Launch faster, cost-effective clinical trials, with full compliance.
Launch faster, cost-effective clinical trials, with full compliance.
Effortlessly design, test, and execute your randomised control trials with intuitive drag-and-drop tools.
Effortlessly design, test, and execute your randomised control trials with intuitive drag-and-drop tools.
Effortlessly design, test, and execute your randomised control trials with intuitive drag-and-drop tools.


Trusted by
What is it like to work with Trial Deck?
What is it like to work with Trial Deck?
The way it works is intuitive and so, as a non-tech savvy person, even I have managed to set up forms with validations and rules in, and then test that they are all fully working. Very impressed!

Johanna Cook
Senior Trial Manager
The way it works is intuitive and so, as a non-tech savvy person, even I have managed to set up forms with validations and rules in, and then test that they are all fully working. Very impressed!

Johanna Cook
Senior Trial Manager
The way it works is intuitive and so, as a non-tech savvy person, even I have managed to set up forms with validations and rules in, and then test that they are all fully working. Very impressed!

Johanna Cook
Senior Trial Manager
The Trial Deck platform is very user-friendly. The blocks within builder mode are easy to configure and the clear layout makes it customisable to suit our data collection objectives

Anthony Quinn
NIHR SMILE Bioresource Project Co-ordinator
The Trial Deck platform is very user-friendly. The blocks within builder mode are easy to configure and the clear layout makes it customisable to suit our data collection objectives

Anthony Quinn
NIHR SMILE Bioresource Project Co-ordinator
The Trial Deck platform is very user-friendly. The blocks within builder mode are easy to configure and the clear layout makes it customisable to suit our data collection objectives

Anthony Quinn
NIHR SMILE Bioresource Project Co-ordinator
Trial Deck has been really helpful in streamlining participant management and data collection for our longitudinal clinical trial. The scheduling features and automated email reminders make the process more efficient and easier to manage.


Elaina Chow
Researcher
Trial Deck has been really helpful in streamlining participant management and data collection for our longitudinal clinical trial. The scheduling features and automated email reminders make the process more efficient and easier to manage.


Elaina Chow
Researcher
Trial Deck has been really helpful in streamlining participant management and data collection for our longitudinal clinical trial. The scheduling features and automated email reminders make the process more efficient and easier to manage.


Elaina Chow
Researcher
Powerful flow builder
Build out trial flows faster than ever before
Recruitment made simple
Collect and securely store patient consent digitally, with support for advanced electronic signatures where required.
Efficient questionnaire builder
Build out complex questionnaires at speed.
Powerful flow builder
Build out trial flows faster than ever before
Recruitment made simple
Collect and securely store patient consent digitally, with support for advanced electronic signatures where required.
Efficient questionnaire builder
Build out complex questionnaires at speed.
Designed for
Clinical Trials Units (CTUs)
Clinical Trials Units (CTUs)
Academic research groups
Academic research groups
Contract Research Organisations (CROs)
Contract Research Organisations (CROs)
Biotech companies
Biotech companies
Everything your team needs to work at speed
Running clinical trials especially large, decentralised ones, can be costly, slow, and complex. Trial Deck simplifies the process, cutting costs and speeding up setup. By streamlining operations and reducing admin work, it lets Trial Managers focus on running successful trials.
Launch in record time
Design and validate clinical trial workflows with ease—use drag-and-drop tools to map participant journeys, annotate in-line, and collaborate in real time.
Launch in record time
Design and validate clinical trial workflows with ease—use drag-and-drop tools to map participant journeys, annotate in-line, and collaborate in real time.


Security first
Trial Deck guarantees compliance with ISO 27001 and NHS DSPT standards. Incorporates advanced security protocols and robust encryption to safeguard patient data.

Security first
Trial Deck guarantees compliance with ISO 27001 and NHS DSPT standards. Incorporates advanced security protocols and robust encryption to safeguard patient data.

Security first
Trial Deck guarantees compliance with ISO 27001 and NHS DSPT standards. Incorporates advanced security protocols and robust encryption to safeguard patient data.
Test, test, test launch
Test, refine, and test again. Launch your trial with confidence using our test center. Simulate and preview the participant experience multiple times to ensure everything runs smoothly.
Test, test, test launch
Test, refine, and test again. Launch your trial with confidence using our test center. Simulate and preview the participant experience multiple times to ensure everything runs smoothly.
Wide ranging API integrations
Easily integrate with the external systems that power your study, from SMS integrations and NHS service lookup to sample logistics and more. Trial Deck is designed with flexibility at its core, offering robust API access to support complex, multi-system workflows across the clinical trial lifecycle.
Wide ranging API integrations
Easily integrate with the external systems that power your study, from SMS integrations and NHS service lookup to sample logistics and more. Trial Deck is designed with flexibility at its core, offering robust API access to support complex, multi-system workflows across the clinical trial lifecycle.
Adverse event reporting
Automatically trigger and record safeguarding events based on researcher defined rules. Whether using PHQ-9 scoring, or a custom questionnaire, alerts are raised when thresholds are met, ensuring timely intervention, accurate record-keep and patient safety.
Adverse event reporting
Automatically trigger and record safeguarding events based on researcher defined rules. Whether using PHQ-9 scoring, or a custom questionnaire, alerts are raised when thresholds are met, ensuring timely intervention, accurate record-keep and patient safety.
Adverse event reporting
Automatically trigger and record safeguarding events based on researcher defined rules. Whether using PHQ-9 scoring, or a custom questionnaire, alerts are raised when thresholds are met, ensuring timely intervention, accurate record-keep and patient safety.
Randomisation made simple
Working alongside study statisticians, we develop bespoke randomisation algorithms tailored to your study's requirements. This includes support for minimisation techniques to ensure balanced participant allocation across key variables.
Randomisation made simple
Working alongside study statisticians, we develop bespoke randomisation algorithms tailored to your study's requirements. This includes support for minimisation techniques to ensure balanced participant allocation across key variables.
Pricing
Pricing
Get a quote in 48 hours
We’re always here to help. Fill out the pricing form by following the link below to get a quote for your study.
We’re always here to help. Fill out the pricing form by following the link below to get a quote for your study.




Elaina Chow
Researcher
Trial Deck has been really helpful in streamlining participant management and data collection for our longitudinal clinical trial. The scheduling features and automated email reminders make the process more efficient and easier to manage.
Trial Deck has been really helpful in streamlining participant management and data collection for our longitudinal clinical trial. The scheduling features and automated email reminders make the process more efficient and easier to manage.
Digital interventions
Fully tailored clinical trials and digital interventions to meet your unique needs
Adapt every aspect of your trial with ease; our team of software engineers will work with you to ensure your trial and digital intervention meet your study’s requirements.
Adapt every aspect of your trial with ease; our team of software engineers will work with you to ensure your trial and digital intervention meet your study’s requirements.
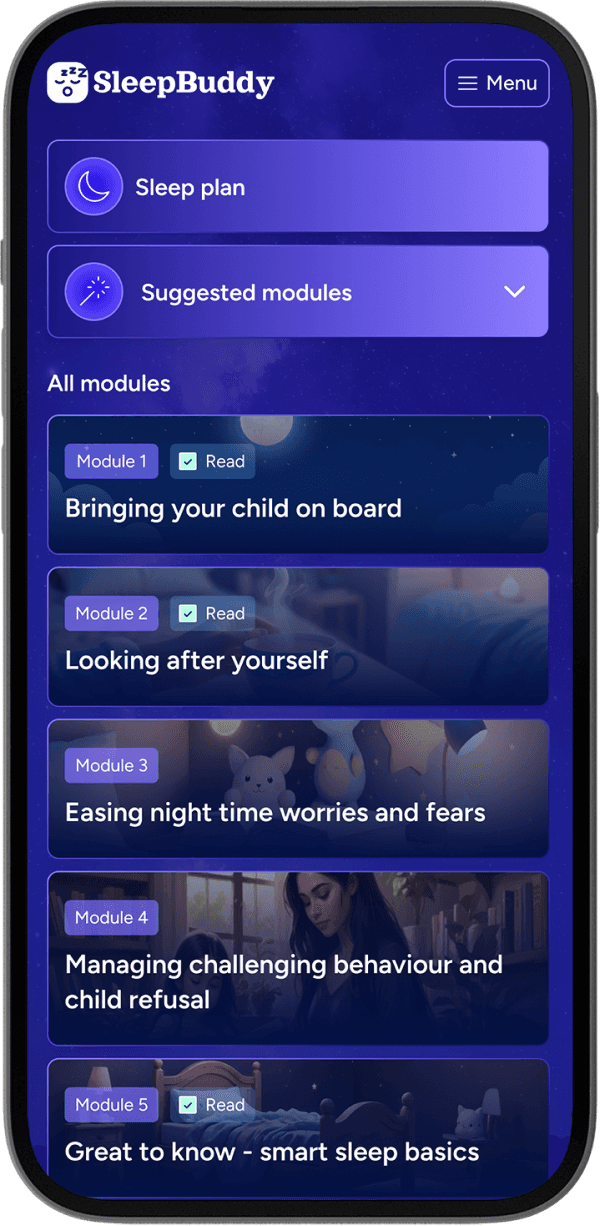
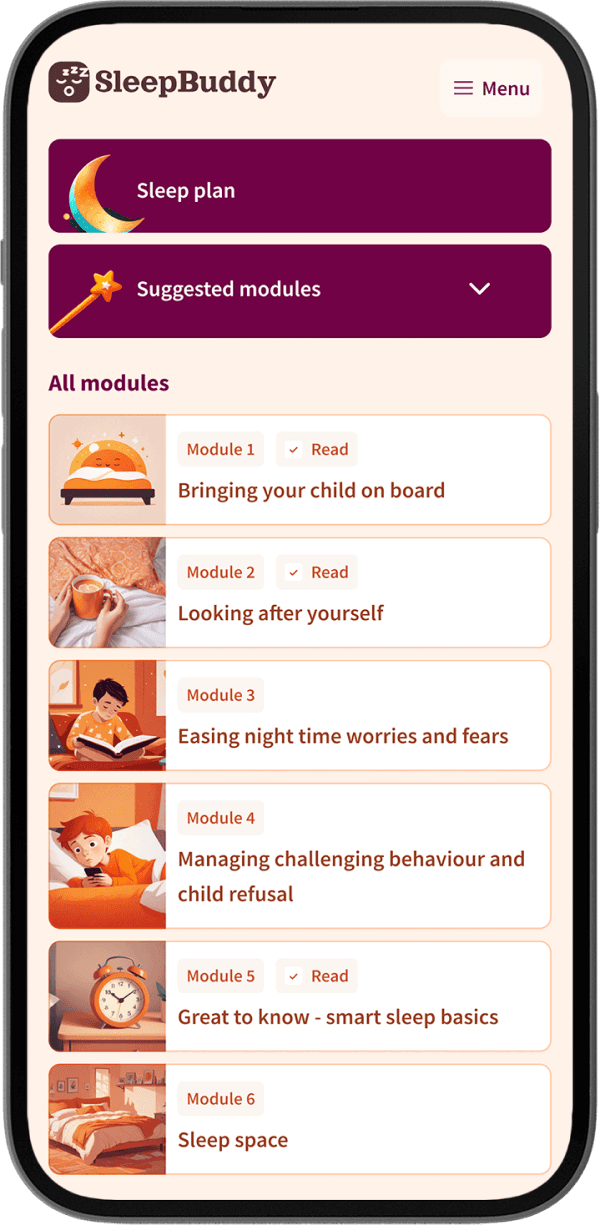
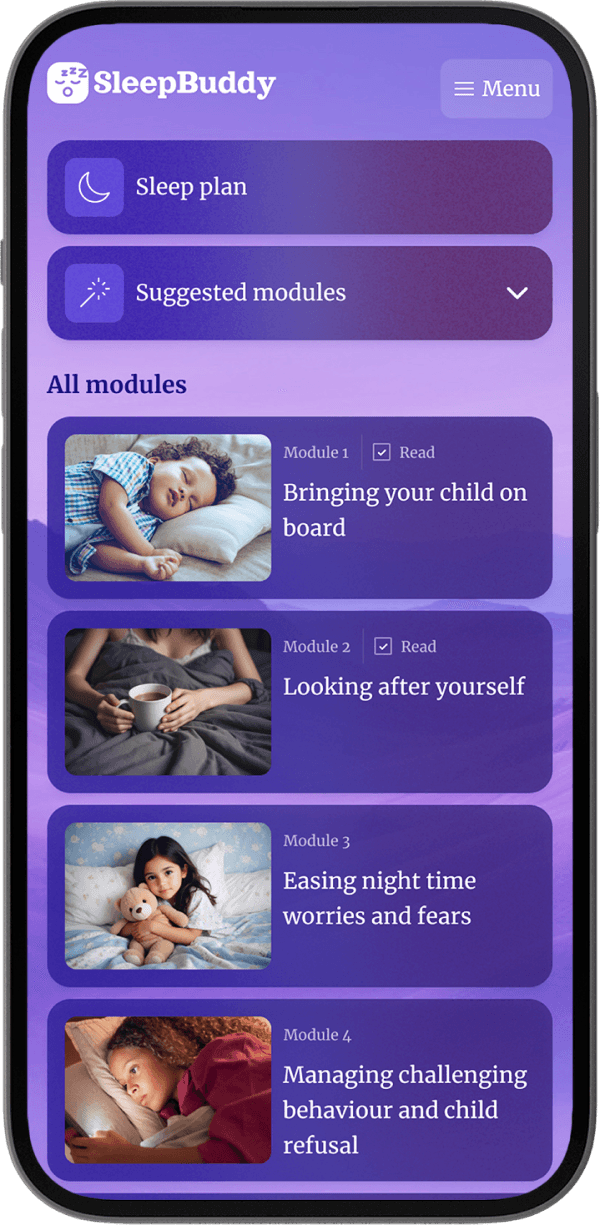
Frequently asked questions.
What is the pricing structure for Trial Deck?
What is the pricing structure for Trial Deck?
Can I name Trial Deck in our grant application?
Can I name Trial Deck in our grant application?
Can I export my participants’ data?
Can I export my participants’ data?
How quickly can I get my trial started on Trial Deck?
How quickly can I get my trial started on Trial Deck?
Can the digital intervention builder help with CBT programmes?
Can the digital intervention builder help with CBT programmes?
Do you offer training on Trial Deck?
Do you offer training on Trial Deck?
Does Trial Deck offer support for decentralised trials?
Does Trial Deck offer support for decentralised trials?
Need more information?
Need more information?
Can’t find the answer you’re looking for? Please chat to our friendly team at enquiries@trial-deck.com for further information.

The latest
Insights and news from Trial Deck
Insights and news from Trial Deck
Let’s Build Better Trials Together
Let’s Build Better Trials Together
You don’t have to choose between innovation and compliance. Between fast delivery and good governance. Between doing the work and proving the work.
You don’t have to choose between innovation and compliance. Between fast delivery and good governance. Between doing the work and proving the work.
Book a demo
Book a demo with one of our directors
Start your free feasibility study
Design your feasibility study for free with Trial Deck
Get a no-obligation quote
Fill out the pricing form
Book a demo
Book a demo with one of our directors
Start your free feasibility study
Design your feasibility study for free with Trial Deck
Get a no-obligation quote
Fill out the pricing form


